OFFICE OF THE ATTORNEY GENERAL
Official Opinion No. 2017-7
RE: Cannabidiol & HEA 1148
EXECUTIVE SUMMARY
The Office of the Indiana Attorney General provides this opinion and information for the public regarding whether it is lawful to sell, use or possess products containing a chemical compound commonly known as cannabidiol.
"CBD" is the scientific abbreviation for a chemical compound known as "Cannabidiol" ("cann-uh-bid-DYE-all"). Cannabidiol is one of the more prevalent chemical compounds in the Cannabis (or Marijuana) plant. The chemical compounds that bind together in an array that constitute cannabidiol are defined as "2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol."1
As a matter of legal interpretation, products or substances marketed for human consumption or ingestion, and containing cannabidiol, remain unlawful in Indiana, and under federal law.2
The state and federal laws that place cannabidiol in the category of a Schedule I controlled substance do not hinge on the degree or prevalence of the pharmacological effects of a substance on a person, for those effects vary from person to person, substance to substance, and component to component. Simply put, cannabidiol is a Schedule I controlled substance because marijuana (Cannabis sativa) is a Schedule I controlled substance. Although it is a relatively new phenomenon, after thoroughly tracking the language of the Indiana law defining "marijuana," it is evident that cannabidiol is now and historically has been derived from "a part of the plant genus Cannabis."3 Scientific literature confirms that cannabidiol simply cannot be distilled in sufficient amounts from any of the inert parts of the plant (such as the "mature stalks of the plant" or the "sterilized seeds of the plant"), Ind. Code 35-48-1-19(b), which are specifically excluded from the basic description of what constitutes "marijuana" according to both state and federal authorities alike.4 Thus, experts and federal authorities are in agreement that mature stalks, sterilized seeds, and other exempt components of the Cannabis plant are insufficient for the manufacture of cannabidiol.5 With that being the case, it is also clear that the parts of the plant being used to manufacture cannabidiol will necessarily include the floral
bracts, resin, and leaves of the Cannabis plant.6
This same analysis has been applied by federal authorities operating under the federal Controlled Substances Act, pursuant to guidance put out by the United States Drug Enforcement Administration ("DEA"), and known as "the Extract Rule."7 Read together, these definitions under Indiana law clearly establish that a "substance containing cannabidiol," being extracted from the resinous (non-inert, non-exempt) parts of the plant genus Cannabis, is "marijuana" and is a Schedule I controlled substance.
Cannabidiol is likely to contain some amount of other cannabinoids (i.e., another basic molecular building-block of Cannabis) such as "Tetrahydrocannabinol," which is better known as "THC."8 THC is known to science and the courts as the key cannabinoid found in marijuana. THC is also the key compound known to produce the euphoric, passive, intoxicated or hallucinogenic state sought after by many who consume marijuana.
Under Indiana law, a "substance containing cannabidiol" is defined to contain a certain amount of cannabidiol (at least 5% by weight) and an amount of THC (not more than 0.3% by weight).9 Under Indiana law, THC, including that found in the "resinous extractives of Cannabis," is itself a Schedule I controlled substance.10 Accordingly, any substance containing cannabidiol is prohibited in Indiana as it falls under the definition of "marijuana" and contains THC, both of which result in it being a Schedule I controlled substance.
In 2017, House Enrolled Act 1148 established a limited and focused exception for possession and use of substances containing cannabidiol by patients and caregivers battling a diagnosis of juvenile or adult treatment-resistant epilepsy.11 According to this legislation, despite the threshold consideration that substances containing cannabidiol retain their prohibition as Schedule I controlled substances, a limited affirmative defense is available. Under this legislatively-created defense, the law makes clear that the burden of proof is on the defendant to show that he qualifies, if he can show that he was properly registered under Indiana's newly created Indiana State Department of Health Cannabidiol Registry ("the Registry").
The existence of the Registry reaffirms that absent this narrowly-focused affirmative defense, substances containing cannabidiol are Schedule I controlled substances, and if identified or so labeled in plain view of a law enforcement officer, are subject to seizure. Individuals possessing or using such substances are subject to prosecution under both state and federal law, barring proof being shown that they qualified for the Registry defense, which under HEA 1148 is limited to those who are battling treatment-resistant epilepsy or their caregivers. Despite the existence of this affirmative defense under HEA 1148, which addresses a kind of medical use and possession by registered, qualified individuals, the Indiana legislature has not yet addressed the distribution or sale of this product. At present, then, no one in Indiana is authorized to commercially distribute or sell CBD or substances containing CBD.
One additional exception allows for limited possession and even cultivation of marijuana under certain narrowly-prescribed circumstances, and that is Indiana's exclusion of "industrial hemp" from its definition of marijuana. On March 26, 2014, Indiana's Industrial Hemp Law ("IHL") was signed by the Governor, and became effective. The IHL classifies industrial hemp as an agricultural product and authorizes the Indiana State Seed Commissioner to pursue the necessary federal permits allowing for the production of, possession of, scientific study of, and limited commerce of industrial hemp. The IHL attempts to make available to Indiana producers the opportunities to cultivate and study industrial hemp afforded by the federal 2014 Farm Bill.12 The federal 2014 Farm Bill created an exception to the federal CSA for the limited growth, cultivation and marketing research experimentation with industrial hemp by an institution of higher education or a State department of agriculture if such activities are conducted within federal legal boundaries.
On August 12, 2016, the United States Department of Agriculture ("USDA"), in consultation with the DEA and the United States Food and Drug Administration ("FDA") published a Statement of Principles on Industrial Hemp ("Statement of Principles") in the Federal Register to inform the public about how Federal law applies to activities associated with industrial hemp. The Statement of Principles reiterates that Section 7606 did not remove industrial hemp from the controlled substances list.
In a statement to the public,13 the DEA further advised that it considers Section 7606 to authorize institutions of higher education and state departments of agriculture to grow and cultivate industrial hemp as defined under the 2014 Farm Bill; however, it does not permit such entities, or anyone else, to produce approved drug products subject to FDA approval for human consumption that are made from Cannabis. This is a direct example of a joint affirmation of federal policy, promulgated by three separate federal agencies, affirming that no industrial hemp pilot program, state or federal, is intended to encompass products for immediate human consumption as food, food supplement, or a drug.
Upon careful study and deliberation, it is the opinion of the Indiana Attorney General that the purchase, possession, use and sale of cannabidiol, and substances, food products or edible oils containing cannabidiol are unlawful under both Indiana and federal law. HEA 1148, as it was intended by the Indiana General Assembly, established a limited affirmative defense for the express purpose of treating those with treatment resistant epilepsy.
BACKGROUND
On April 26, 2017, the Honorable Governor Eric J. Holcomb signed HEA 114814 (codified at Ind. Code § 16-42-28.6 et seq.) into law, which permits the legal use of a substance containing cannabidiol for the treatment of epilepsy under limited conditions.15 Under the new law, the Indiana State Department of Health ("ISDH") is authorized to develop and implement a cannabidiol registry of patients and caregivers for the use of a substance containing cannabidiol in the treatment of patients who have been diagnosed with treatment-resistant epilepsy.16
Although the provisions of Ind. Code § 16-42-28.6 et seq. allow for the use and possession of a substance containing cannabidiol by properly registered caregivers and patients, the law does not contemplate, or expressly establish, a regulatory scheme allowing for the actual manufacture, distribution, or sale of cannabidiol and substances containing cannabidiol in Indiana. Despite permitting limited use and possession of substances containing cannabidiol by properly registered individuals, the purchasing or otherwise actually obtaining lawful access to substances containing cannabidiol remains highly problematic, and has not been adequately addressed under Indiana law. Currently, Ind. Code § 16-42-28.6 et seq. only describes the limited circumstances under which use and possession of cannabidiol is legal in Indiana.
ANALYSIS
In Indiana, the primary goal of a court when construing a statute is to ascertain the legislative intent.17 "To discern that intent, [an Indiana court will] look first to the statutory language itself and give effect to the plain and ordinary meaning of statutory terms."18 Our Indiana Supreme Court summarized the starting point for statutory interpretation as follows: "[i]f a statute is unambiguous, that is, susceptible to but one meaning, we must give the statute its clear and plain meaning. However, if a statute admits of more than one interpretation, then it is ambiguous; and we thus resort to rules of statutory interpretation so as to give effect to the legislature's intent."19
The language of HEA 1148, first and foremost, authorizes the limited use and possession of a substance containing cannabidiol by caregivers and patients properly registered with the ISDH.20 In doing so, the legislature enacted an affirmative defense for possession and use of these substances for properly registered individuals. Accordingly, this permitted use must be understood in the context of existing statutes regulating and prohibiting the use of controlled substances in Indiana, as codified in Ind. Code § 35-48-1-2 to Ind. Code § 35-48-7-14. The analysis below demonstrates that "cannabidiol" and "substance containing cannabidiol" are prohibited by the Indiana Controlled Substances Act with a limited exception for those permitted under HEA 1148. In considering the implications of the Indiana Controlled Substances Act, it is necessary to examine the application of Indiana's industrial hemp exception as it relates to "substance containing cannabidiol." But the analysis cannot stop there. It is also prudent to consider the impact of federal law and regulations concerning marijuana and industrial hemp given the federal government's authority to enforce the federal Controlled Substances Act (CSA), 21 U.S.C. § 801, et seq.
I. HEA 1148 Created a Limited Affirmative Defense.
Pursuant to Ind. Code § 16-42-28.6-1, "cannabidiol" is defined as "2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol." Additionally, Ind. Code § 16-42-28.6-5 defines "substance containing cannabidiol" as follows:
Ind. Code 16-42-28.6-5 "Substance containing cannabidiol"
. . . a product that contains:
(1) not more than three-tenths percent (0.3%) total tetrahydrocannabinol (THC) by weight, including its precursors and derivatives;
(2) at least five percent (5%) cannabidiol by weight; and
(3) no other controlled substances.
Despite Ind. Code § 16-42-28.6 et seq. authorizing the use and possession of a substance containing cannabidiol by caregivers and patients properly registered with the ISDH, it does not exclude these substances from other regulations and classifications related to narcotic and nonnarcotic drugs. In fact, HEA 1148 only authorized an affirmative defense to certain offenses and conditions. In relevant part, HEA 1148 authorized the following affirmative defense to Ind. Code § 35-48-4-11 (possession of marijuana, hash oil, hashish, or salvia):
(d) It is a defense to a prosecution under subsection (a)(1) based on the possession of a substance containing cannabidiol that:
(1) the person is a patient or caregiver registered under
IC 16-42-28.6 for the use of a substance containing cannabidiol;
(2) the person reasonably believed that the substance possessed by the person was a substance containing cannabidiol; and
(3) the substance containing cannabidiol is packaged in a container labeled with the origin, volume, and concentration by weight of total THC, including its precursors and derivatives, and cannabidiol.[21]
As such, it is presumed under Indiana law that anyone claiming to possess these items has an affirmative duty to prove they are in compliance with the law.22 To the extent that a substance does not satisfy the definition of "cannabidiol" or "substance containing cannabidiol," it is not permissible under Ind. Code § 16-42-28.6 et seq. and no affirmative defense could be proven. For example, the provisions of Ind. Code § 16-42-28.6 et seq. are not implicated when a substance contains more than three-tenths percent (0.3%) total Tetrahydrocannabinol (THC) by weight or contains less than five percent (5%) cannabidiol by weight.
II. Apart from the limited exception in HEA 1148, "cannabidiol" and "substance containing cannabidiol" are prohibited by the Indiana Controlled Substances Act.
Given that a "substance containing cannabidiol"–as defined to include Tetrahydrocannabinol–would not be excluded from the definition of "marijuana" under Ind. Code § 35-48-1-19, individuals in possession of these substances without meeting the proper conditions are in violation of both state and federal law. Under the Indiana Controlled Substances Act,23 the legislature has determined that substances listed in statutory Schedules I through V are considered controlled substances.24 The Indiana Controlled Substances Act further provides that a "controlled substance" is defined to include a drug, substance, or immediate precursor of a drug listed in those schedules and penalty groups.25 The Schedule I classification includes certain hallucinogenic substances.26 For these hallucinogenic substances, Ind. Code § 35-48-2-4(d) specifies that any material, compound, mixture, or preparation containing any quantity of the substance listed under Subsection (d)(1)-(d)(45)–including their salts, isomers, and salts of isomers–are considered a Schedule I controlled substance. This list of substances includes "Tetrahydrocannabinols" which is a potential ingredient for "substance containing cannabidiol" under HEA 1148. "Tetrahydrocannabinols" is defined in the list of Schedule I controlled substances under Ind. Code § 35-48-2-4 as the following:
Ind. Code 35-48-2-4 Schedule I
. . .
(31) Tetrahydrocannabinols (7370), including synthetic equivalents of the substances contained in the plant, or in the resinous extractives of Cannabis, sp.[27] and synthetic substances, derivatives, and their isomers with similar chemical structure and pharmacological activity such as:
. . .
Since nomenclature of these substances is not internationally standardized, compounds of these structures, regardless of numerical designation of atomic positions are covered. Other name: THC.
Based on the statutory definition, a "substance containing cannabidiol" may contain Tetrahydrocannabinol, which results in it being a Schedule I controlled substance that contains synthetic equivalents or resinous extractives from the Cannabis plant for purposes of the Indiana Controlled Substances Act.
What is more, by specifying that these substances contain material that is derived from non-exempt parts of the Cannabis plant–namely cannabidiol–the legislature classified these substances within the realm of offenses related to marijuana. Regardless of the presence of any THC, cannabidiol is prohibited by the Indiana Controlled Substances Act since it is included in the definition of marijuana. Although neither the definition of "cannabidiol" nor "substance containing cannabidiol" directly reference marijuana or the Cannabis plant, the new statutory provisions contemplate cannabidiol as it relates to marijuana given that cannabidiol is derived from non-exempt parts of the plant genus Cannabis.28
For purposes of the Indiana Controlled Substances Act, the term "marijuana" is defined in Ind. Code § 35-48-1-19 as follows (emphasis supplied):
Sec. 19. (a) "Marijuana" means any part of the plant genus Cannabis whether growing or not; the seeds thereof; the resin extracted from any part of the plant, including hashish and hash oil; any compound, manufacture, salt derivative, mixture, or preparation of the plant, its seeds or resin.
(b) The term does not include:
(1) the mature stalks of the plant;
(2) fiber produced from the stalks;
(3) oil or cake made from the seeds of the plant;
(4) any other compound, manufacture, salt, derivative, mixture, or preparation of the mature stalks (except the resin extracted therefrom);
(5) the sterilized seed of the plant which is incapable of germination; or
Because "cannabidiol" is derived from non-exempt parts of the plant genus Cannabis, it squarely falls under the statutory definition of marijuana.30 Moreover, given that a "substance containing cannabidiol" is defined to contain Tetrahydrocannabinols which contain synthetic equivalents or resinous extractives of the Cannabis plant, any substance containing cannabidiol would also fall under the statutory definition of marijuana. Yet, the statute also provides exceptions to the definition of "marijuana" under Subsections (b)(1) through (b)(6). To determine whether any of these exceptions apply to a substance containing cannabidiol, it is necessary to consider which part of the Cannabis plant these substances are derived from most commonly.
The chemical compounds of cannabidiol–defined as "2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol" in Ind. Code § 16-42-28.6-1–and Tetrahydrocannabinol are most commonly derived from the resinous floral bracts and leaves of the Cannabis plant.31 In fact, it is well established that cannabidiol cannot be meaningfully produced from the plants' seeds or stalks.32 While small amounts of the cannabinoid may be found on the stalk of certain varieties of the Cannabis plant, the greatest concentrations of cannabidiol are found in the floral bracts and leaves of the plant.33 This understanding is consistent with the DEA's analysis that "cannabinoids are not found in the parts of the Cannabis plant that are excluded from the [federal] CSA definition of marijuana, except for trace amounts (typically, only parts per million)" and "based on the scientific literature, it is not practical to produce extracts that contain more than trace amounts of cannabinoids using only the parts of the Cannabis plant that are excluded from the [federal] CSA definition of marijuana[.]"34 As such, the only possible exception to exclude cannabidiol from the definition of "marijuana" would be based on whether either substance could be considered "industrial hemp" as defined by Ind. Code § 15-15-13-6.
a. Indiana's industrial hemp exception does not apply to "cannabidiol" or "substance containing cannabidiol."
In 2014, the Honorable Governor Michael R. Pence signed SEA 35735 into law, authorizing the Indiana State Seed Commissioner ("Commissioner") to license the cultivation and production of industrial hemp under Indiana's Industrial Hemp Law ("IHL").36 Additionally, the legislation required the Commissioner to apply for any necessary permissions, waivers, or other forms of legal status by the DEA before January 1, 2015, in order to implement the new statutory provisions regarding industrial hemp.37 Until permission is granted from federal authorities, neither individuals nor companies are permitted to grow industrial hemp or possess its seeds in Indiana.38 As discussed in further detail infra, federal authorities have only granted limited authorization of industrial hemp as of the date of this opinion. In fact, Section 7606 of the 2014 federal Farm Bill–which provided federal legal framework for Indiana's Industrial Hemp Law39–clearly limits the cultivation of hemp for research purposes to institutions of higher education and state departments of agriculture.40
For purposes of licensing the cultivation and production of industrial hemp, the term "industrial hemp" is defined in Ind. Code § 15-15-13-6 as follows:
Sec. 6. As used in this chapter, "industrial hemp" means:
(1) all nonseed parts and varieties of the Cannabis sativa plant, whether growing or not, that contain a crop wide average tetrahydrocannabinol (THC) concentration that does not exceed the lesser of:
(A) three-tenths of one percent (0.3%) on a dry weight basis; or
(B) the percent based on a dry weight basis determined by the federal Controlled Substances Act (21 U.S.C. 801 et seq.); or
(2) any Cannabis sativa seed that is:
(A) part of a growing crop;
(B) retained by a grower for future planting; or
(C) for processing into, or use as, agricultural hemp seed.
The term does not include industrial hemp commodities or products.[41]
Based on Subsection (1) of this definition, it is possible that a substance containing cannabidiol could be considered a substance that contains "industrial hemp." Both definitions include either a direct or indirect reference to substances derived from a Cannabis plant, and both definitions limit the amount of Tetrahydrocannabinol in the substance to not more than three-tenths percent (0.3%) by weight. However, the last line of Ind. Code § 15-15-13-6 specifies that "industrial hemp" is not defined to include the commodities or products of industrial hemp.
The term "industrial hemp commodities" as used in Ind. Code § 15-15-13-6 is not defined, and the more generic term "commodity"42 is also not defined anywhere under Title 15. Black's Law Dictionary defines "commodity" broadly as "[a]n article of trade or commerce."43
It is plausible that one could argue that the limitation on the amount of Tetrahydrocannabinol in both Ind. Code § 15-15-13-6(1) and Ind. Code § 16-42-28.6-5(1) means that, by definition, a "substance containing cannabidiol" could be derived from "industrial hemp," and therefore it could be considered an industrial hemp product or commodity. Yet, that conclusion provides little utility here. Even if a substance containing cannabidiol would be considered derived from industrial hemp, it is not covered under Indiana's Industrial Hemp Law due to the express exclusion of "industrial hemp commodities or products."44 Accordingly, it is not exempt from the definition of "marijuana" under the Indiana Controlled Substances Act.
The language of the HEA 1148 is very specific and limited. Any argument of a contrary legislative intent is without merit. First, the legislature created a very specific limited exception to cannabidiol, in which it is an affirmative defense to the possession of cannabidiol. The fact that the legislature made it an affirmative defense and not an exception under the definition of marijuana evinces the legislature's choice to define a substance containing cannabidiol as "marijuana" and make it a defense if the individual possessing the substance satisfied certain conditions. If the legislature had intended to legalize cannabidiol under the IHL then the recent law would not have been necessary. Furthermore, one cannot reasonably argue that cannabidiol was intended to be available for all children regardless of any medical condition and completely without regulation. The plain and ordinary meaning of the language simply does not support that expansive understanding. Recognizing that it is unnecessary to the conclusions above, the current status of federal law and regulations over marijuana and industrial hemp further informs this analysis of HEA 1148.
III. HEA 1148 does not affect enforcement of the federal Controlled Substances Act (CSA), 21 U.S.C. § 801, et seq.
The provisions under Ind. Code § 16-42-28.6, et seq. do not diminish the federal authority to enforce federal laws relating to marijuana regardless of state law. The DEA is the federal agency in charge of enforcing the federal Controlled Substances Act (CSA), 21 U.S.C. § 801, et seq.45 The CSA creates five schedules of substances. Numerous natural and synthetic substances are placed on one of these five schedules based upon assessments of the substances' medical uses, abuse potential, safety issues or tendency toward causing dependence on the part of the user. Generally speaking, a substance and a product that can be derived from that substance are listed in the same schedule.46
a. The definition of "marijuana" under the federal CSA includes "cannabidiol" and "substance containing cannabidiol" as defined by Indiana.
The CSA definition of "marijuana" specifically includes "all parts of the plant Cannabis sativa L., whether growing or not; the seeds thereof; the resin extracted from any part of such plant; and every compound, manufacture, salt, derivative, mixture, or preparation of such plant, its seeds or resin."47 As one advances through the process of definition, a series of exclusions and exemptions come into play under the statutes; these will be discussed in greater detail, infra.
Plants grown to produce marijuana as a drug and plants grown to produce hemp products both derive from plants belonging to the genus Cannabis.48 The government's long-held view is that Cannabis is a single, polytrophic genus that can manifest differing characteristics. Varieties can therefore be developed through hybridization to exhibit desired attributes, such as low-Tetrahydrocannabinol forms or high-cannabidiol forms. This is why the United States Congress, federal enforcement authorities, and courts traditionally have held fast to the botanical characterization of all parts of the Cannabis plant identified in the definition under the CSA as marijuana. 49
In December 2016, the DEA promulgated a final rule establishing a new drug code 7350 for "Marihuana extract" under Schedule I of the CSA.50 Since cannabidiol is extracted from the prohibited parts of the Cannabis plant–specifically, the buds, leaves, and resins extracted from non-sterile seeds–cannabidiol is a prohibited marijuana extract.51
The described approach delivers us squarely to the conclusion that cannabidiol is illegal under federal law, and under the application of state law.52 For purposes of the DEA classification system of illicit drugs, botany is the distinction. The issue is not whether cannabidiol, standing alone, creates a psychotropic effect in the user. The issue for purposes of the CSA classification comes down to whether the substance is derived from the genus Cannabis and parts of the plant that are within the CSA definition of "marijuana."
b. The federal exemption for "industrial hemp" is not applicable for "cannabidiol" and "substance containing cannabidiol" as defined by Indiana.
On February 4, 2014, the U.S. Congress approved the Agricultural Act of 2014,53 otherwise known as the "2014 Farm Bill."
The 2014 Farm Bill defines "industrial hemp" as:
"[T]he plant Cannabis sativa L. and any part of such plant, whether growing or not, with a THC concentration of not more than 0.3 percent on a dry weight basis."54
Section 7606 amounts to an exception to the CSA for the limited growth, cultivation and marketing research experimentation for industrial hemp by an institution of higher education or a state department of agriculture, if such activities are conducted within federal legal boundaries. Furthermore, Section 7606 authorized state departments of agriculture to promulgate regulations necessary to carry out these pilot programs; however, it did not provide specific delegation to the USDA or any other agency to implement the program.
On August 12, 2016, the USDA, in consultation with the DEA and the FDA published a Statement of Principles on Industrial Hemp ("Statement of Principles") in the Federal Register to inform the public about how federal law applies to activities associated with industrial hemp grown and cultivated in accordance with Section 7606. The Statement of Principles reiterate that Section 7606 did not remove industrial hemp from the controlled substances list: ". . . Federal law continues to restrict hemp-related activities, to the extent that those activities have not been legalized under Section 7606."55
Additionally, the Statement of Principles outlines nine principles to inform educational institutions and state governments that want to participate in industrial hemp agricultural pilot programs in accordance with federal law. One of these principles specifies that for purposes of marketing research (including distribution of marketing materials) under Section 7606, industrial hemp products may only be sold in a state with an agricultural pilot program; however, these products may not be sold or exchanged in states where sales are otherwise prohibited. 56 Notably, the IHL expressly excludes "industrial hemp commodities or products" from its definition of "industrial hemp."57 Moreover, marketing research may not be for the purpose of general commercial activity, and industrial hemp plants and seeds may not be transported across state lines.
In a statement to the public,58 the DEA further advised that it considers Section 7606 to authorize institutions of higher education and state departments of agriculture to grow and cultivate industrial hemp as defined under the 2014 Farm Bill; however, it does not permit such entities, or anyone else, to produce approved drug products subject to FDA approval for human consumption that are made from the Cannabis plant. This is a direct example of a joint affirmation of federal policy, promulgated by three separate federal agencies, affirming that no industrial hemp pilot program, state or federal, is intended to encompass products for immediate human consumption as food, a food supplement, or a drug.
For all of the reasons stated above, possession of cannabidiol remains prohibited under federal law, regardless of the circumstances, and is therefore potentially subject to seizure by any law enforcement agency.
IV. Seizures are permissible when a substance containing cannabidiol is in plain view.
In view of the foregoing analysis, which concludes that a substance containing cannabidiol is legally categorized under state and federal law as marijuana, questions arise as to the what appropriate actions may be taken by responsible law enforcement officials. The Fourth Amendment of the Constitution of the United States allows the warrantless seizure of contraband when three factors are met: 1) the officer must be rightfully occupying the location; 2) the item must be in plain view; and, 3) the incriminating nature of the evidence must be immediately apparent.59 Under this standard, the rationale is that if contraband is left in open view and is observed by a police officer from a lawful vantage point, there has been no search within the meaning of the Fourth Amendment.60
Article I, Section 11 of the Indiana Constitution requires a different analysis that focuses on the reasonableness of police conduct, which is analyzed by balancing: 1) the degree of concern, suspicion, or knowledge that a violation has occurred; 2) the degree of intrusion the method of the search or seizure imposes on the citizens' ordinary activities; and (3) the extent of law enforcement needs.61
Illegal cannabidiol products, openly for sale at a number of traditional retail stores and by other commercial channels throughout the state, can be characterized as illegal contraband, subject to warrantless seizure under the Fourth Amendment of the Constitution of the United States and Article I, Section 11 of the Indiana Constitution. Especially since no one is authorized to distribute or sell cannabidiol in Indiana under either federal or state law.
An entire stock or inventory of products clearly labeled as containing cannabidiol, that are lawfully in the plain view of a police officer, are subject to warrantless seizure. Testing of the products is not a precondition to the seizure. If subsequent testing should find that products were mislabeled as to the ingredients contained therein, or that they actually contain no cannabidiol whatsoever, Ind. Code §§ 35-48-4-4.5, 35-48-4-4.6, and 35-48-4-5 provide for the prosecution of certain offenses related to substances that are represented to be a controlled substance.
CONCLUSION
We understand that cannabidiol is a substance about which we know very little, and about which many hold out a good deal of hope that it may be that elusive cure for any number and kind of disease. One hopes, for the sake of those who are suffering any of these maladies, that it can be so. But hoping and wishing are not the proper role of government. Our job is to place our fellow citizens fairly on notice about what the laws passed by their representatives really mean.
As the analysis represented by this opinion has shown, the legal conclusion is that cannabidiol is illegal to buy, sell, possess or use in the State of Indiana. Yes, a narrowly drawn exception exists in the form of an affirmative defense for properly registered caregivers and children suffering from treatment-resistant epilepsy. This affirmative defense would only apply to prosecutions under state law. However, they may still be subject to federal prosecution. Therefore, anyone possessing a substance containing cannabidiol, or anything packaged as such, in plain view of a law enforcement officer is subject to having that property seized. Only upon a showing that said individual meets the limited conditions under Indiana law, could that individual not be prosecuted under state law. Furthermore, no one in Indiana is authorized to sell cannabidiol under either federal or state law, and therefore, any retail establishment selling anything that contains cannabidiol is in violation of the law.
SUBMITTED, and
ENDORSED FOR PUBLICATION:
Curtis T. Hill, Jr.
Attorney General
Scott C. Newman
Chief Counsel, Advisory Division
___________________________
1 Ind. Code § 16-42-28.6-1.
2 This conclusion does not apply to any product that is approved by the FDA. There are currently two products that contain cannabidiol undergoing clinical trials; Epidiolex and Sativex.
3 Ind. Code 35-48-1-19(a).
4 Id.; see also H. Mölleken & H. Hussman,
Cannabinoid in seed extracts of Cannabis sativa cultivars, J. Int. Hemp Assoc. 4(2): 73-79 (1997); S. Ross et al.,
GC-MS Analysis of the Total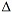 9-THC Content of Both Drug- and Fiber-Type Cannabis Seeds
9-THC Content of Both Drug- and Fiber-Type Cannabis Seeds, 24 J. Anal. Toxic. 715-717 (2000).
5 DEA Diversion Control Division, Clarification of the New Drug Code 7350 for Marijuana Extract, available at https://www.deadiversion.usdoj.gov/schedules/marijuana/m_extract_7350.html (last visited November 20, 2017).
7 Establishment of a New Drug Code for Marihuana (sic) Extract, 80 Fed. Reg. 90,194-196 (Dec. 14, 2016), codified at 21 C.F.R. § 1308.11(d)(58).
8 There is some dispute among the manufacturers and producers of substances containing cannabidiol products as to whether these products can be produced without containing "THC." Under the current state of the law in Indiana, it is not necessary that the substance contain "THC" for the substance to be illegal. If it is derived from the floral bract or leaves of the plant, regardless of the presence of "THC," it is illegal. See Ind. Code § 35-48-1-19 (definition of "marijuana" does not require it to contain THC).
9 Ind. Code § 16-42-28.6-1.
10 Ind. Code § 35-48-2-4.
11 The provisions of HEA 1148 are codified at Ind. Code §§ 16-42-28.6 et seq.
12 See Ind. Code §15-15-13, et seq.
13 DEA Statement on CBD, Hemp and "Farm Bill" (Aug. 2015).
14 House Enrolled Act 1148 (P.L.188-2017).
15 Ind. Code § 16-42-28.6-5.
16 Ind. Code § 16-42-28.6-7; Under Ind. Code § 16-42-28.6-6, "treatment resistant epilepsy" is defined to include: (1) Dravet syndrome; (2) Lennox-Gastaut syndrome; or (3) another form of epilepsy in a patient who has not responded to at least two (2) other epilepsy treatment option that have been provided in good faith.
17 Adams v. State, 960 N.E.2d 793, 798 (Ind. 2012).
18 Pierce v. State, 29 N.E.3d 1258, 1265 (Ind. 2015).
19 Suggs v. State, 51 N.E.3d 1190, 1193–94 (Ind. 2016) (internal citations omitted).
20 Ind. Code § 16-42-28.6 et seq.
21 Ind. Code § 35-48-4-11(d).
22 See, Newson v. State, 785 N.E.2d 1155, 1157 (Ind. Ct. App. 2003).
23 Ind. Code § 35-48-1-2 to Ind. Code § 35-48-7-14.
24 Ind. Code § 35-48-1-9.
25 Ind. Code § 35-48-1-9.
26 Ind. Code § 35-48-2-4(d).
27 "Cannabis, sp." means any species of the plant genus Cannabis, including Cannabis sativa and Cannabis indica.
28 Ind. Code § 16-42-28.6-5; see also H. Mölleken & H. Hussman,
Cannabinoid in seed extracts of Cannabis sativa cultivars, J. Int. Hemp Assoc. 4(2): 73-79 (1997); S. Ross et al.,
GC-MS Analysis of the Total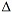 9-THC Content of Both Drug- and Fiber-Type Cannabis Seeds
9-THC Content of Both Drug- and Fiber-Type Cannabis Seeds, 24 J. Anal. Toxic. 715-717 (2000).
30 H. Mölleken & H. Hussman,
Cannabinoid in seed extracts of Cannabis sativa cultivars, J. Int. Hemp Assoc. 4(2): 73-79 (1997); S. Ross et al.,
GC-MS Analysis of the Total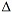 9-THC Content of Both Drug- and Fiber-Type Cannabis Seeds
9-THC Content of Both Drug- and Fiber-Type Cannabis Seeds, 24 J. Anal. Toxic. 715-717 (2000).
31 Galovski, Austin. Marijuana's Impact on California High Intensity Drug Trafficking Report 2016. United States: Smart Approaches to Marijuana (SAM, 2016), p. 53.; see also Karl W. Hillig & Paul G. Mahlberg, A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae), American Journal of Botany, June 2004, vol. 91 no. 6, 966-975.
34 DEA Diversion Control Division, Clarification of the New Drug Code 7350 for Marijuana Extract, available at https://www.deadiversion.usdoj.gov/schedules/marijuana/m_extract_7350.html (last visited November 20, 2017).
35 Senate Enrolled Act 357 (P.L.165-2014).
36 P.L.165-2014 (SEA 357).
37 Ind. Code § 15-15-13-15.
39 Ind. Code § 15-15-13-1 ("Nothing in this chapter authorizes any person to violate any federal law or regulation.")
40 § 7606 of the 2014 Farm Act, Agricultural Act of 2014, Pub. L. No. 113-79, 128 Stat. 912-13, (codified, as amended, at 7 U.S.C.A. § 5940 (2017)).
42 However, Ind. Code § 15-16-5-1 does define the term "agricultural commodity" to mean "any plant or part of a plant and animals or animal products produced primarily for sale, consumption, propagation, or other use by humans or animals." While other provisions–such as under Ind. Code § 15-15-13-7–further specify that industrial hemp is an agricultural product, the exception under the Indiana Controlled Substances Act for the definition of "marijuana" is specific only to the provisions under Ind. Code § 15-15-13-6.
43 BLACK'S LAW DICTIONARY (10th ed. 2014).
44 Ind. Code § 15-15-13-6.
45 The DEA's rules and regulations enforcing the CSA are located under 21 C.F.R. Part 1308.
46 21 U.S.C. § 812(c), Schedules I-V.
47 21 U.S.C. § 802(a)(16).
48 The scientifically accepted means of classifying all life forms is tied to the notion of "binomial nomenclature." Under this convention, all life is classified by the organism's membership in narrowing categories known as Kingdom, Phylum, Class, Order, Family, Genus, and Species. Cannabis is the genus common to all marijuana and hemp plants, though the species may vary between them.
49 See, U.S. v. Sanapaw, 366 F.3d 492, 495 (7th Cir. 2004) (citing to numerous other courts and stating that "[i]t is absurd to believe that Congress intended to ban the euphoric effect of one species of marijuana but not the exact same euphoric effect of other species of marijuana . . .").
50 Establishment of a New Drug Code for Marihuana (sic) Extract, 80 Fed. Reg. 90,194-196 (Dec. 14, 2016), codified at 21 C.F.R. § 1308.11(d)(58).
51 DEA Diversion Control Division, Clarification of the New Drug Code 7350 for Marijuana Extract, available at https://www.deadiversion.usdoj.gov/schedules/marijuana/m_extract_7350.html (last visited November 20, 2017).
52 The United States Food and Drug Administration (FDA) which is authorized to regulate and support scientific research on the potential therapeutic uses of marijuana compounds, 21 U.S.C. §§ 301, et seq., as amended, also determined that "products containing [cannabidiol] are outside the definition of a dietary supplement." FDA Inspections, Compliance, Enforcement, and Criminal Investigations, Warning Letter, Sana Te 2/4/16, https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2016/ucm484987.htm (last visited September 11, 2017). No cannabidiol-related product has received FDA approval. Id.
53 Agricultural Act of 2014, Pub. L. No. 113-79, 128 Stat. 912-13, (codified, as amended, at 7 U.S.C.A. § 5940 (2017)).
54 § 7606 of the 2014 Farm Act, Agricultural Act of 2014, Pub. L. No. 113-79, 128 Stat. 912-13, (codified, as amended, at 7 U.S.C.A. § 5940 (2017)).
55 81 Fed. Reg. 53,395 (Aug. 12, 2016).
57 Ind. Code § 15-15-13-6.
58 DEA Statement on CBD, Hemp and "Farm Bill" (Aug. 2015) (on file with the authors).
59 See, Wilkinson v. State, 70 N.E.3d 392, 402 (Ind. Ct. App. 2017) (citing Jones v. State, 783 N.E.2d 1132, 1137 (Ind. 2003)).
60 See, Minnesota v. Dickerson, 508 U.S. 366, 375 (1993).
61 See, Wilkinson, 70 N.E.3d at 405.
Posted: 11/29/2017 by Legislative Services Agency
DIN: 20171129-IR-010170555AOA
Composed: Apr 27,2024 12:04:16AM EDT
A
PDF version of this document.

